Skyrizi Package Insert 2025
Skyrizi Package Insert 2025. Risankizumab as induction therapy for crohn’s disease: D’haens g, panaccione r, baert f, et al.
1 indications and usage 1.1 plaque psoriasis. Skyrizi is an intravenous infusion given every 4 weeks x 3 doses indicated for the treatment of moderately to severely active crohn’s disease.
Injection Instructions & Tutorial Skyrizi Complete, Gordon kb, strober b, lebwohl m, et al. Always speak to your health provider about the risks and benefits of a drug.
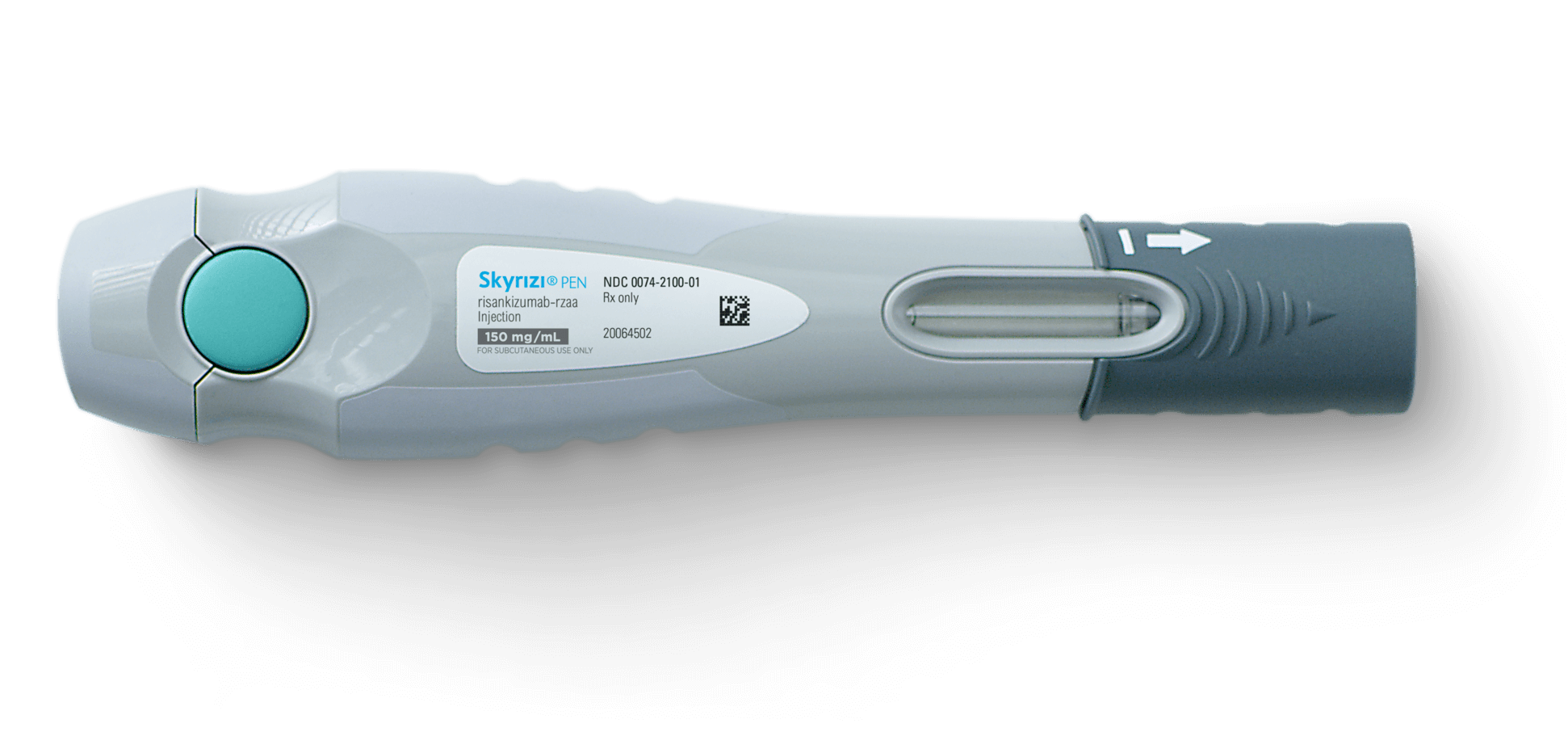
Skyrizi Injection FDA prescribing information, side effects and uses, 1 indications and usage 1.1 plaque psoriasis. Skyrizi is an intravenous infusion given every 4 weeks x 3 doses indicated for the treatment of moderately to severely active crohn’s disease.
SKYRIZI TV Spot, 'Downtown Getaway' iSpot.tv, For healthcare provider administration only. Refer to the skyrizi package insert for complete information.

Skyrizi Commercial (2025) YouTube, Food and drug administration (fda) has approved skyrizi ®. Risankizumab as induction therapy for crohn’s disease:

skyrizi event on Behance, 1 indications and usage 1.1 plaque psoriasis. 180 mg/1.2 ml (150 mg/ml) as a colorless to yellow, and clear to slightly opalescent solution.

SKYRIZI® (risankizumabrzaa) Dosing Schedule & Injection Support, Refer to the skyrizi package insert for complete information. A biologic treatment for adult patients living with moderate to severe plaque psoriasis, adults with active psoriatic arthritis, and adults with moderately to severely active crohn's disease.
Contact us Success, Gordon kb, strober b, lebwohl m, et al. Gordon kb, strober b, lebwohl m, et al.

SKYRIZI® (risankizumabrzaa) Receives FDA Approval, Gordon kb, strober b, lebwohl m, et al. Talk to your doctor about the options available to.

How to pronounce skyrizi, 180 mg/1.2 ml (150 mg/ml) as a colorless to yellow, and clear to slightly opalescent solution. Abbv) today announced the u.s.

skyrizi event on Behance, Refer to the skyrizi package insert for complete information. Abbv) and allergan aesthetics, an abbvie company, today announced they will present 29.
